The fourth amendment to China’s Patent Law (Article 42) introduced for the first time the patent term extension regime in China.
The newly revised Implementing Regulations of the Patent Law and the Guidelines for Patent Examination, which will enter into force on January 20, 2024, flesh out the much-needed details of such regime.
Article 42 of the Patent Law prescribes two types of patent term extension: 1) extension to compensate for unreasonable delay in the prosecution process of an invention, which is the Chinese counterpart of the US PTA mechanism (PTA); and 2) extension to compensate for the time required for obtaining administrative approval to market new drug, which is the Chinese counterpart of the US PTE mechanism (PTE).
PTA 101 in China
Invention patents that have experienced unreasonable delay in the prosecution process are eligible for PTA, unless the applicant files on the same day an invention patent and a utility model for the same invention, and the invention patent is later granted (Article 9 of the Patent Law).
In principle, the request for PTA shall be made by the patentee to the CNIPA within 3 months from the date of grant. In the transitional period between the entry-into-force of the fourth amendment of the Patent Law (June 1, 2021) and that of the newly revised regulations (January 20, 2024), invention patent granted after June 1, 2021 is eligible for PTA (whose examination commences after January 20, 2024), provided that the PTA application is submitted within 3 months from the date of grant. Where a patent, which is eligible for PTA, has expired before January 20, 2024, a PTA can still be granted, with the extended term commencing on the original expiry date.
The extended term of PTA shall be calculated as follows:
Extended term = unreasonable delay in the prosecution process – reasonable delay – unreasonable delay caused by the applicant
-
- Unreasonable delay in the prosecution process
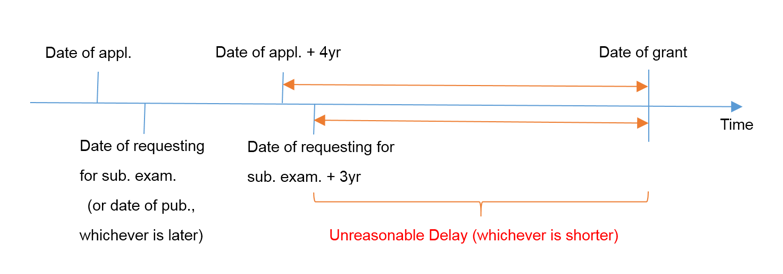
An unreasonable delay in the prosecution process refers to the undue delay in the date of grant. It is defined as the interval between “4 years after the date of application”, “3 years after the date of requesting for substantial examination”, “3 years after the date of publication”, whichever is later, and the date of grant.
For PCT applications and divisional applications, the date of entry into national phase and the date of filing divisional application shall be deemed as the date of application respectively.
- Reasonable delay
Reasonable delay shall be deducted from the extended term. The regulations enumerate below circumstances qualifying as reasonable delay:
-
- where the applicant has revised the application documents in filing the re-examination request or in response to the notification of re-examination in re-examination proceeding;
- where the examination has been suspended due to the disputes over the right to file a patent application;
- where the court has ruled to take preservation measures against the right to file a patent application; and
- other eligible circumstances.
- Unreasonable delay caused by the applicant
Unreasonable delay caused by the applicant (as shown below) shall also be deducted from the extended term:
-
- where the applicant has failed to respond to an office action in due time;
- where the applicant has requested for deferral of examination;
- where the applicant has requested incorporation by reference;
- where the applicant has requested restoration of right; or
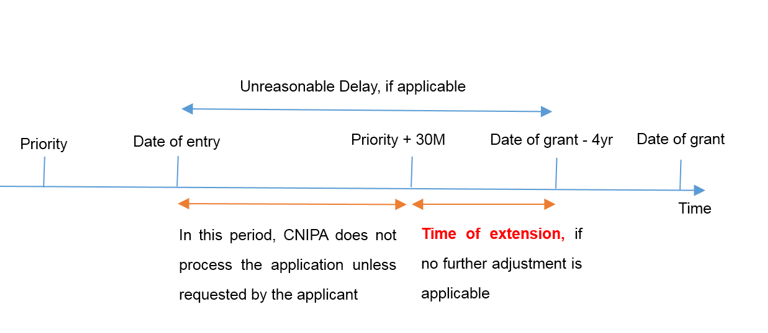
- where the applicant has not requested to process a PCT application prior to the expiration of 30 months from the priority date.
Article 23 of PCT prescribes that a designated Office shall not process or examine the international application prior to the expiration of 30 months from the priority date, in absence of an explicit request of the applicant. Where the applicant does not make such a request, the interval between the date of entry into national phase and the expiration of 30 months from the priority date shall be deducted from the extended term.
- Examination and Remedy
The examination of PTA shall follow the principle of hearing, i.e., the applicant shall be given at least one opportunity to make observations and/or to amend the documents.
The patentee and the third party that is involved in a patent infringement dispute may apply to the CNIPA for administrative reconsideration against a decision to grant/not to grant a PTA.
PTE 101 in China
Invention patents seeking protection over products, methods of preparation or medical uses (Swiss-type), which are associated with approved innovative drugs or certain modified new drugs are eligible for PTE.
In accordance with the drug registration classification system of China’s National Medical Products Administration (NMPA), patents associated with drugs in the following categories are eligible for PTE:
- For TCM (traditional Chinese medicine) drugs or natural drugs:
-
- innovative drugs;
- modified new drugs treating new indications.
- For chemical drugs:
-
- innovative drugs that have not yet been marketed in China or overseas;
- modified new drugs that is the esterification or salification of known active ingredients;
- modified new drug treating new indications.
- For biologicals:
-
- innovative vaccines and innovative biologicals for therapeutic purpose;
- modified vaccines utilizing new virus seed;
- modified biologicals treating new indications.
It is worth noting that a novel drug which have been marketed overseas before filing application for marketing approval in China will not be deemed as an “innovative drug”, and the pertinent patents are therefore not eligible for PTE in China.
On top of that, a patent that is eligible for PTE shall meet the following conditions:
-
- the date of grant of the patent predates the date of obtaining the marketing approval of the drug implementing the said patent;
- the patent is valid;
- the patent term has not been extended by PTE;
- a claim of the patent incorporates the technical solution of a drug that has obtained marketing approval;
- where the drug implements multiple patents, only one patent may be granted PTE;
- where a patent is implemented by multiple drugs, application for PTE may be filed for only one of the drugs.
- Application Time Limit
The request for PTE shall be made by the patentee to the CNIPA within 3 months from the date of obtaining the marketing approval. In case the patentee is not simultaneously the holder of the marketing approval, a written consent of the latter shall be obtained.
During the PTE term of a patent, the protection scope of such patent shall be limited to the technical solutions of the approved new drug and the approved indications.
In the transitional period, a patent for which a PTE application is filed after June 1, 2021, is eligible for PTE, provided that the PTE application is filed within 3 months from the date of obtaining the marketing approval of the drug implementing such patent.
- Calculation of the Extended Term
In practice, the patentee may request both PTA and PTE, should his invention experienced unreasonable delay in the prosecution process, and the said patentee went through a lengthy procedure to obtain administrative approval to market new drugs implementing such invention. In such case, the PTE term shall be determined on the basis of the PTA term, unless the patentee explicitly waives the PTA.
The term of PTE, which is subject to the provisions of Article 42(3) of the Patent Law, is determined by deducting 5 years from the interval between the filing date of the patent and the date of obtaining the new drug marketing approval in China. Specifically, the extended term is capped at 5 years, with the total validity period after obtaining the marketing approval capped at 14 years.
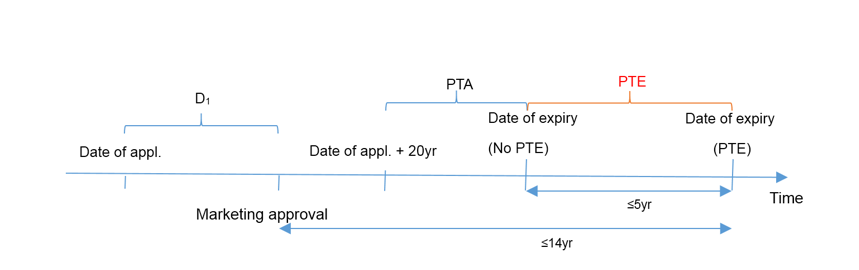
We could observe from the above time axis that:
-
- PTE = the time interval between the date of application and the date of obtaining marketing approval (D1) – 5 years
- PTE ≤ 5 years
- interval between the date of obtaining marketing approval and the original date of expiry (20 – D1) + PTA + PTE ≤ 14 years
We would be able to deduce that:
-
- if D1-PTA≤6 years, no PTE could be obtained;
- if 6 years<D1-PTA≤11 years, the term of PTE equals D1- PTA -6 years;
- if D1-PTA>11 years, the term of PTE equals to 5 years.
- Examination and Remedy
The examination and remedy of PTE is analogous to that of PTA, except that the party which is eligible to apply for an administrative reconsideration also include a third party that has filed an application to obtain the marketing approval for a relevant drug.
Comment
It remains to be seen how the PTA and PTE regime will be implemented in China. The Innovative and generic drug makers are advised to watch closely on the CNIPA’s case law to see if the agency will provide any further guidance on this matter.